Scientists Develop Artificial Leaf That Uses Sunlight to Produce Valuable Chemicals
Decades of research leads to self-contained solar panels that convert carbon dioxide into C2 products
Decades of research leads to self-contained solar panels that convert carbon dioxide into C2 products
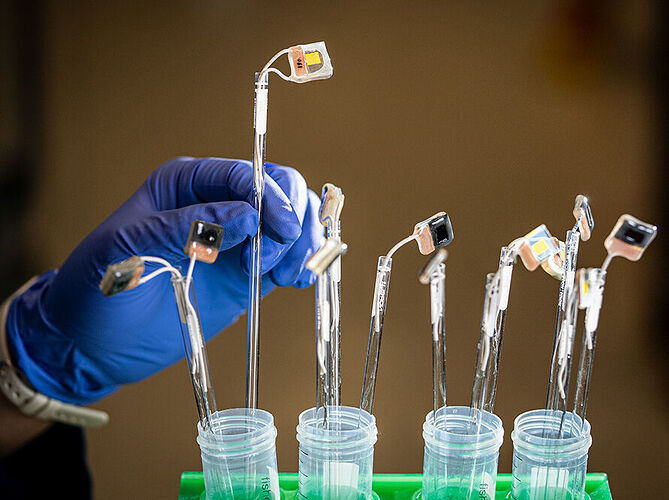
Peidong Yang (right) and Jia-An Lin, a graduate researcher, used lead halide perovskite photoabsorbers, which imitate a leaf’s light absorbing chlorophyll, and electrocatalysts made of copper that resemble tiny flowers. (Credit: Marilyn Sargent/Berkeley Lab)
Key Takeaways
- The Liquid Sunlight Alliance is a multi-institutional collaboration working to develop the tools needed to use energy from sunlight to produce liquid fuels.
- Researchers built a perovskite and copper-based device that converts carbon dioxide into C2 products – precursory chemicals of innumerable products in our everyday lives, from plastic polymers to jet fuel.
- This proof-of-concept research opens new opportunities for energy research.
Researchers from the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) along with international collaborators have brought us one step closer to harnessing the sun’s energy to convert carbon dioxide into liquid fuel and other valuable chemicals. In a recent publication in Nature Catalysis , the researchers debut a self-contained carbon-carbon (C2) producing system that combines the catalytic power of copper with perovskite, a material used in photovoltaic solar panels. This advance builds on over 20 years of research and brings the scientific community one step closer to replicating the productivity of a green leaf in nature.
This work is part of a larger initiative, the Liquid Sunlight Alliance (LiSA), which is a Fuels from Sunlight Energy Innovation Hub funded by the U.S. Department of Energy. Led by Caltech in close partnership with Berkeley Lab, LiSA brings together more than 100 scientists from national lab partners at SLAC and the National Renewable Energy Laboratory, and university partners at UC Irvine, UC San Diego, and the University of Oregon. Researchers involved in this multi-institutional collaboration have made advances in developing our understanding of and the tools needed to develop liquid fuels generated from sunlight, carbon dioxide, and water. (Learn more about the LiSA collaboration in this roundup, “Five Ways LiSA is Advancing Solar Fuels.”)
Closeup of the perovskite and copper-based devices developed by a multi-institutional collaboration working to develop the tools needed to turn sunlight into liquid fuel. (Credit: Marilyn Sargent/Berkeley Lab)
Artistic depiction of an artificial tree with copper nanoflowers wired to perovskite crystals.
Artistic depiction of an artificial tree with copper nanoflowers wired to perovskite crystals. (Credit: Virgil Andrei)
“Nature was our inspiration,” said Peidong Yang, a senior faculty scientist in Berkeley Lab’s Materials Sciences Division and UC Berkeley professor of chemistry and materials science and engineering involved in the published work. “We had to work on the individual components first, but when we brought everything together and realized that it was successful, it was a very exciting moment.”
To build a system that mimics photosynthesis, Yang and his team followed the natural processes that occur in the leaf of a plant. Each individual component of a leaf’s photosynthesizing elements had to be replicated and refined. Tapping into the decades’ worth of research, the scientists used lead halide perovskite photoabsorbers to imitate a leaf’s light-absorbing chlorophyll. And inspired by enzymes that regulate photosynthesis in nature, they designed electrocatalysts made of copper that resemble tiny flowers.